Experiment 1: Production of an acidic solution by contact of water with pyrite
Material:
- Beaker
- Glass rod or spoon
- Pyrite pebbles
- Distilled water
For the following experiment, we will first prepare an acidic water sample. To do this, we proceed as in the experiment “Acid Rock Drainage.” We will now prepare a larger amount of sulfuric acid water.
- Pour 50 ml of distilled water into a beaker.
- Then add some pyrite stones into the beaker so that the bottom is covered, and stir.
- Now wait 10-15 minutes, stirring occasionally.
Versuch 2: Dissolving Copper Ions from Malachite
Material:
- Beaker
- Glass rod
- Malachite (quartz)
- Water from Experiment 1
- The solution from Attempt 1 is brought into contact with some pieces of malachite in a beaker.
- To do this, pour the solution over the stones.
- After 10 minutes, you can use the water for Attempt 3.

Experiment 3: Determination of Heavy Metal Content Using a Photometer and a Calibration Curve
Material:
- Beaker
- 2 test tubes with screw caps
- Test tube rack
- Colorimeter
- Glass rod or spoon
- Pyrite pebbles
- Malachite (quartz)
- Test reagents
- Copper indicator
- Distilled water
- Ammonia solution
- Copper sulfate solution of known concentration
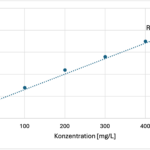
Here, we will create our own calibration curve and use ammonia to form a colored complex.
- First, prepare a dilution series of copper sulfate in the test tubes. You should create at least 3 (preferably more) different concentrations ranging from 0 to 500 mg/L.
- Next, add ammonia drop by drop to the prepared dilutions until the color no longer changes.
- Then, place the samples one by one into the colorimeter/photometer. For the Vernier colorimeter, set the wavelength to 610 nm (for other photometers/colorimeters, choose a wavelength in the range of 600 nm).
- Create a calibration curve from the data. You can use a spreadsheet program to do this.
- Using this calibration curve, you can then measure your sample from the malachite.